Common methods of extracting Hemp Oil
Ethanol is one of many naturally occurring alcohols, compounds that contain a hydroxyl group (OH). Ethanol is produced naturally by the fermentation of sugars or starches. It is the same alcohol that is found in our adult beverages (beer, wine, and hard liquors). Aside from its long history of consumption for intoxication, ethanol is also an effective solvent in extraction, and has been used for thousands of years by cultures all around the world for both of these purposes. It is a polar molecule, meaning it has both positively and negatively charged elements (hydrogen & oxygen respectively). Thus, ethanol binds to the water-soluble and insoluble compounds in hemp, and results in the extracted oil having a wider range of plant compounds than one could derive with other solvents. This includes waxes, chlorophyll, fatty acids, and lipids as well as the more common compounds such as the cannabinoids and terpenes. It’s these attributes that makes ethanol ideal for producing a true Full Spectrum oil and subsequent products. This is the only method we have and continue to use.
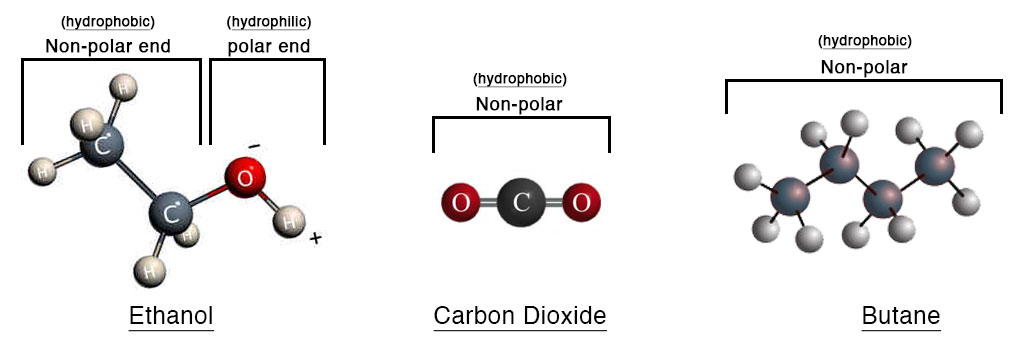
Carbon Dioxide or CO2 is a naturally occurring molecule that is produced as a result of decaying organic materials, fermentation byproduct, or combustion of organic compounds. Most recently CO2 is noted for its negative effect on our atmosphere particularly in its connection to fossil fuel use. In the realm of extraction, CO2 can be used to bind to compounds in the plant material. Used originally for coffee decaffeination, and vanilla extraction, CO2 extraction has become an adopted method to extract oil from hemp. In order to achieve this, CO2 gas must be compressed and cooled. This renders the gas into liquid phase making it useful as a solvent in which to “wash” the hemp material. This process is referred to as “sub-critical” or “supercritical” CO2 extraction.
As far as solvents for plant oil extraction, CO2 is the most efficient per amount of plant material. Due to CO2 ‘s small molecular size and high binding capacity (to some plant compounds), a lot less is required to extract a particular amount of hemp than would be ethanol, butane, or lipid solvents. This is why many large scale extraction facilities prefer CO2 as it is much more cost effective and is able to process large amounts of hemp quickly. However, the fundamental caveat to this is that CO2 is a non-polar molecule and is unable to bind to, and thus extract, a significant amount of available plant compounds compared to ethanol. The goal in any plant extraction is for the solvent to bind to the target molecules of the plant, therefore removing them from the biomass and containing them within the solvent. The solvent is then filtered from the biomass, and finally the solvent removed resulting in a concentrate of solely plant compounds.
Other common solvents worth noting in hemp extraction, though used less commonly than the aforementioned methods, are Butane and Lipid (Oils – commonly MCT, Olive, or Hemp). Although these solvents are moderately effective in binding to the compounds in a plant they are also both non-polar like CO2, and incapable of producing a true Full Spectrum oil or product. There is some concern of residual solvents in the end CBD product, particularly with butane. Ethanol, CO2, and lipids are considered safe and are all in our kitchen in one way or another. Lastly, it is worth mentioning lipid extraction which generally uses applied heat, agitation, and a lipid solvent such as plant oils to bind to compounds in hemp. Oil in itself is not a very good solvent. It is a large molecule comparatively, and a strong non-polar compound so this process relies solely on heat and agitation to separate molecules from the plant and is probably the most inefficient method of extraction.






